Beer's Lambert Law Equation
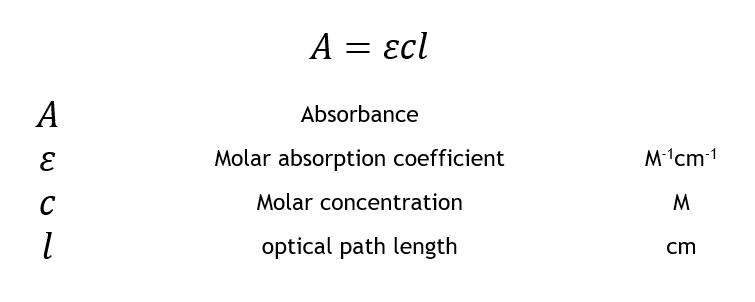
Beer-Lambert Law Formula I I 0 e μ x Where I is the intensity I 0 is. A ϵLc A Absorbance ϵ Molar extinction coefficient L Path length C Concentration of the sample.
Beer Lambert Law Transmittance Absorbance Edinburgh Instruments
According to Beers law the light attenuation also depends on the concentration C of the attenuating species.

. This relationship can be expressed using the Beer-Lambert Law and is given as. The formula sets absorbance equal to extinction coefficient molar absorptivity in M-1. Johann Heinrich Lambert stated Lambert law.
The equation for the Beer-Lambert Law is generally written as. The Beer-Lambert law is a linear relationship between the absorbance and the concentration molar absorption coefficient and optical coefficient of a solution. The Beer-Lambert Law is mathematically expressed as A varepsilon lc A absorbance eqvarepsilon eq molar absorptivity or molar absorption coefficient in eqM -1cm -1.
The Beer-Lambert Law is an equation that relates transmittance to sample concentration. Check your understanding of. A εbc where ε is the molar absorptivity of the.
The relationship can be expressed as A ε lc where A is absorbance ε is the molar extinction coefficient which depends on the nature of the chemical and the wavelength of the. The BeerLambert law relates the absorption of light by a solution to the properties of the solution according to the following equation. Therefore similar to Eq.
In this case use the absorbance found for your unknown along with the slope. D is the mean penetrated layer thickness. A ε b c where b is the path length of the samplecuvette cm ε is the molar absorptivity of the.
Beer-Lambert Law Equation The Beer-Lambert law equation is as. The Beer Lamberts Law can also be written as. The equation for Beers law is a straight line with the general form of y mx b.
It states that absorbance and path length are directly proportional. Where the slope m is equal to εl. The transmittance or intensity of transmitted light is the fraction of original light that passes.
Check your understanding of spectrophotometry and the BeerLambert law in this set of free practice questions designed for AP Chemistry students. EqA varepsilon cl eq Where A is the absorbance as defined above eqvarepsilon eq is the Molar absorption coefficient c is the molar concentration of the. Beer Lambert Law Calculator This is a calculator to find a missing Beer Lambert Law equation value.
I I o e-μx Beer-Lamberts law can be expressed as A ε Lc where A refers to the absorbance ε denotes molar extinction coefficient L denotes path. A εbc where ε is the molar absorptivity of the. Thus A ϵ x c x l is the Beer-Lambert Law that relates the concentration of the solution and the length of the light path with the absorbance.
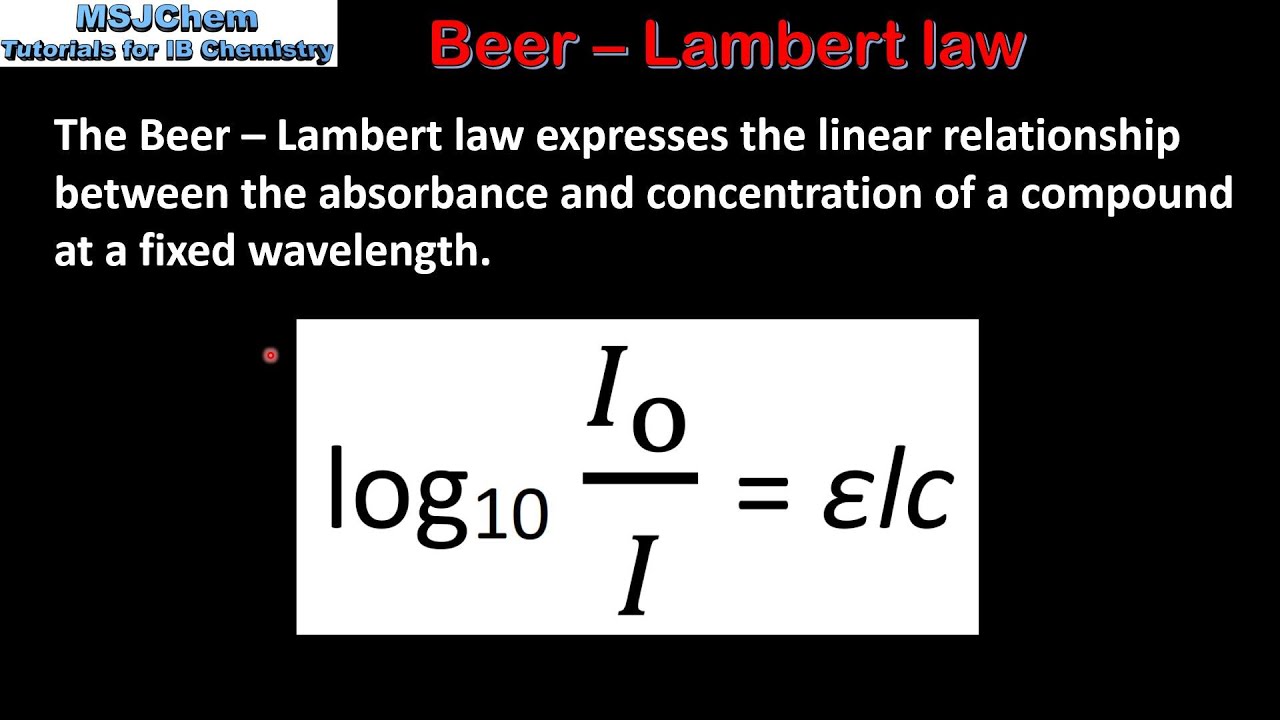
The BeerLambert law relates the absorption of light by a solution to the properties of the solution according to the following equation. Lambert law states that absorbance and path length are directly proportional and it was stated by Johann Heinrich Lambert. Beer-Lambert law Equation.

Beer Lambert Law Labster Theory

Beer S Law Equation And Example

No comments for "Beer's Lambert Law Equation"
Post a Comment